

Others embrace the AAHA recommendation to give the vaccine every three years.
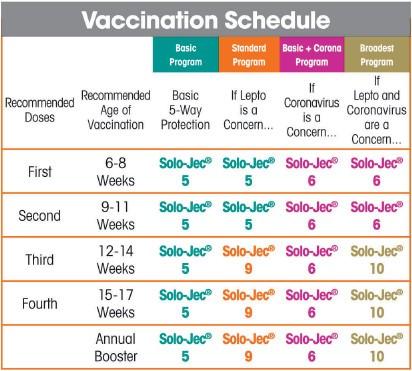
Some veterinarians are uncomfortable extending the vaccine interval past the traditional one year. The distemper combination vaccines are different. They are usually given once to puppies, boosted a year later, and then administered every three years. The requirement for rabies has not changed. Canine Spectra KC3 is 3-way protection in healthy dogs 8 weeks of age or older against Bordetella bronchiseptica (B.bronch), canine parainfluenza (CPIV), and canine adenovirus type 2 (CAV2). Though they may prevent shedding of the organism in the urine, they do not prevent infection.įrequency of revaccination has been a hot topic for several years. Giardia vaccines are also not endorsed by the AAHA. Leptospiral vaccines have a low efficacy (less than 75 percent), and most do not provide protection against the strains that currently cause disease in dogs. Even when the disease does occur, it is mild and self-limiting. For example, too few dogs succumb to coronavirus to justify vaccination. If this weren’t controversial enough, they stated that some vaccines are not recommended. Fill Spectra Vaccine Record, Edit online. These are only to be used where exposure to the disease is likely. The AAHA assigned parainfluenza, bordetella, and Lyme disease to the noncore group. For the vaccination of healthy, susceptible dogs and puppies as an aid in the reduction of diseases caused by Canine Distemper, Canine Adenovirus Types 1 & 2. The AAHA panel agreed that four core vaccines should be administered to every dog: In 2006 they reconfirmed these recommendations. The AAHA also recommended moving the “annual” vaccine to every three years. It suggested that a few vaccines are absolutely necessary, but some are only required in unique circumstances, and others should not be given at all. The American Animal Hospital Association (AAHA) made headlines in 2003 when it published its vaccination recommendations. Consult your vet about your dog’s vaccinations. For advice on revaccination frequency and annual booster vaccinations, consultation with a veterinarian is recommended.Opinions differ over the necessity of some dog vaccinations and the timeframe for vaccinating your dog. The need for this booster has not been established. Historically, annual revaccination with a single dose has been recommended for this product. The presence of maternal antibody is known to interfere with the development of active immunity in dogs and additional boosters will be required in most young animals. All dogs should initially receive one dose of this product and a second dose 2 to 3 weeks later. If blood enters the syringe freely, choose another injection site. IMPORTANT NOTE: Before injecting vaccine, pull back slightly on syringe plunger. Do not inject directly into blood vessel (see note below).
CANINE SPECTRA 5 VACCINATION SHEET SKIN
Subcutaneous administration: Lift the loose skin behind the neck or behind the front leg and insert needle (see illustration, arrows 1 and 2). Withdraw entire contents into the syringe. Aseptically rehydrate the vaccine cake with 1 mL of sterile diluent supplied. Gentamicin added as preservative.ĭirections and dosage: Open the syringe by twisting or tapping the cap against a hard surface to break the heat weld. HE - 06007-5 ( SSS ) PAGE, IRVINE H, Cleveland Clinic Fdn, Cleveland. This product contains a CPV2b strain which has been demonstrated effective against disease caused by CPV2c in puppies with maternal antibody. Study of vaccines for respiratory illness in children Fed Proc 23:40, 64. For more information regarding efficacy and safety data go to .Ĭomposition: Canine Spectra ® 5 vaccine is a combination of immunogenic, attenuated strains of CDV, CAV2, CPIV, and CPV type 2b, propagated in cell line tissue cultures The CAV2 fraction has been shown to be effective against disease due to CAV1. The duration of immunity of this product has not been determined. Indications: This product has been shown to be effective for the vaccination of healthy dogs 6 weeks of age or older against canine distemper virus (CDV), infectious canine hepatitis (CAV1), canine adenovirus type 2 (CAV2), canine parainfluenza (CPIV), and canine parvovirus (CPV). Canine Distemper-Adenovirus Type 2-Parainfluenza-Parvovirus Vaccine


 0 kommentar(er)
0 kommentar(er)
